Clavicle fractures account for approximately 5% of all fractures in adults. They are particularly common in young active adults and athletes, often resulting from high-energy trauma (traffic accidents or sports-related injuries), but also occur in patients over 70 years old following low-energy trauma (fall from standing height). They are classified by location into three segments: the lateral third, the middle third, and the medial third.
The Alians Clavicle S range offers a variety of plates adapted to many types of fractures, whether simple or complex. It allows treatment from the medial to the lateral third, via superior or anterior approaches, thanks to a complete range of associated implants.
The implants in the Alians Clavicle S range are dedicated to the fixation of fractures, delayed unions, pseudarthroses, and clavicle osteotomies in adults.
A comprehensive solution for managing clavicle fractures,
from the medial third to the lateral third.
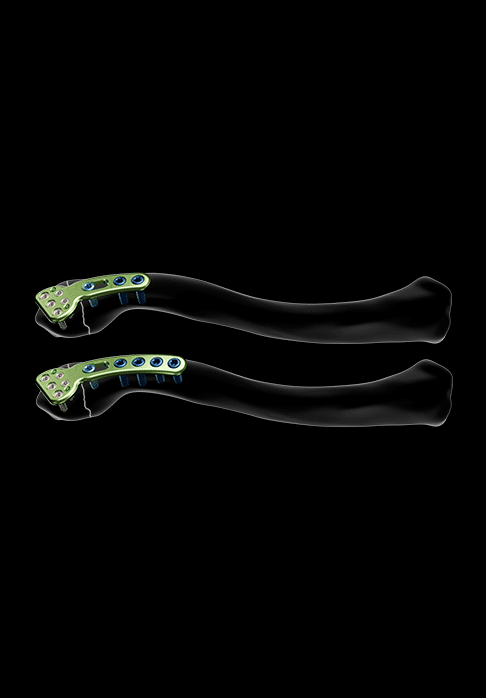
A model of plates, asymmetrical and available in sizes 1 and 2, is offered for the treatment of extra-lateral fractures.
They notably feature:
- Polyaxial pegs for locked Ø2.8 mm screws (in the epiphyseal area)
- Oblong peg for non-locked Ø3.5 mm screws
- Pegs for locked Ø3.5 mm screws and non-locked Ø3.5 mm screws (in the diaphyseal area)
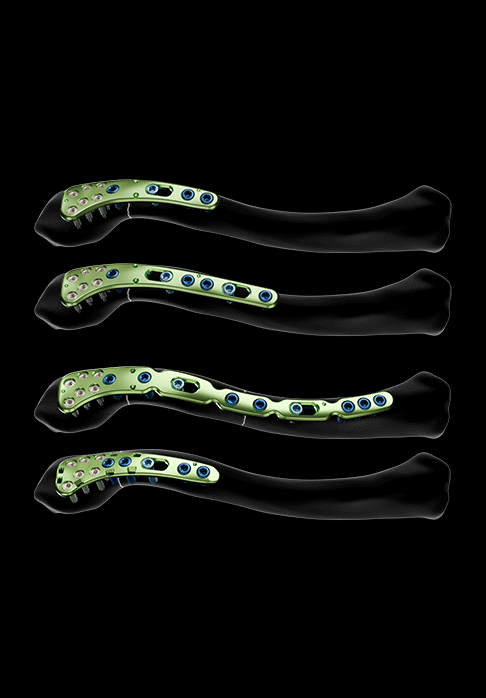
Three plate models are offered for the treatment of lateral third fractures:
- A non-malleable lateral plate model, asymmetrical and available in sizes 1 and 2
- A malleable lateral plate model, asymmetrical and available in size 1, allowing for better bone adaptation in cases of complex fractures and revision surgery for pseudarthrosis
- A lateral plate model with suture holes, asymmetrical and available in size 1, allowing for additional fixation of coracoclavicular or acromioclavicular disjunctions using suture thread
These three plate models feature:
- Polyaxial holes for Ø2.8 mm locking screws (in the epiphyseal area)
- Oblong holes for Ø3.5 mm non-locking screws
- Holes for Ø3.5 mm locking screws and Ø3.5 mm non-locking screws (in the diaphyseal area)
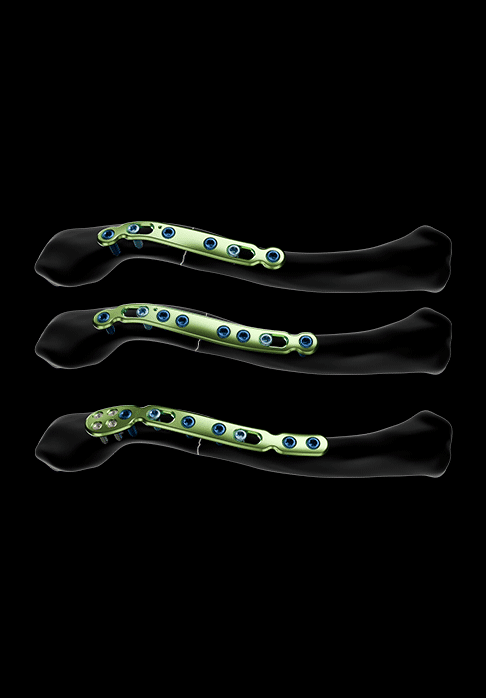
Two plate models are offered for the treatment of middle third lateral fractures:
- A non-malleable middle third lateral plate model, asymmetrical and available in sizes 1 and 2
- A malleable middle third lateral plate model, asymmetrical and available in size 1:
- Featuring a bending zone, it allows better bone adaptation in cases of complex fractures or revision surgery for
pseudarthrosis - Equipped with lateral polyaxial holes for Ø2.8 mm locking screws
- Featuring a bending zone, it allows better bone adaptation in cases of complex fractures or revision surgery for
Both plate models also feature:
- Oblong holes for Ø3.5 mm non-locking screws
- Holes for Ø3.5 mm locking screws and Ø3.5 mm non-locking screws
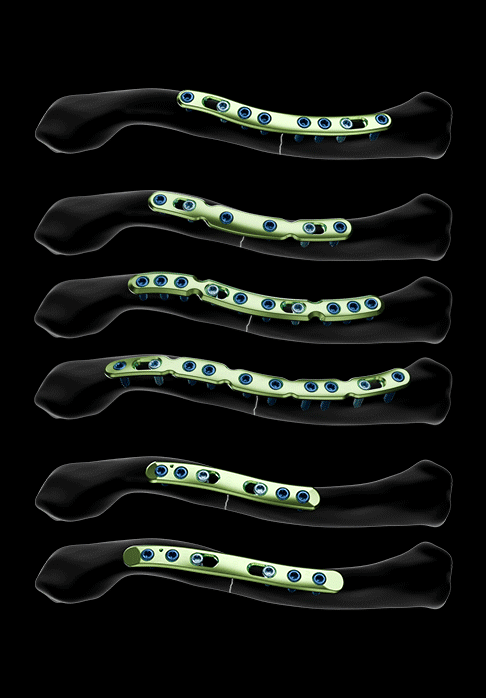
Two plate models are offered for the treatment of middle third fractures:
- A non-malleable middle third plate model, asymmetrical and available in 3 sizes
- A malleable middle third plate model, asymmetrical and available in 3 sizes
Both plate models feature:
- Oblong holes for Ø3.5 mm non-locking screws
- Holes for Ø3.5 mm locking screws and Ø3.5 mm non-locking screws
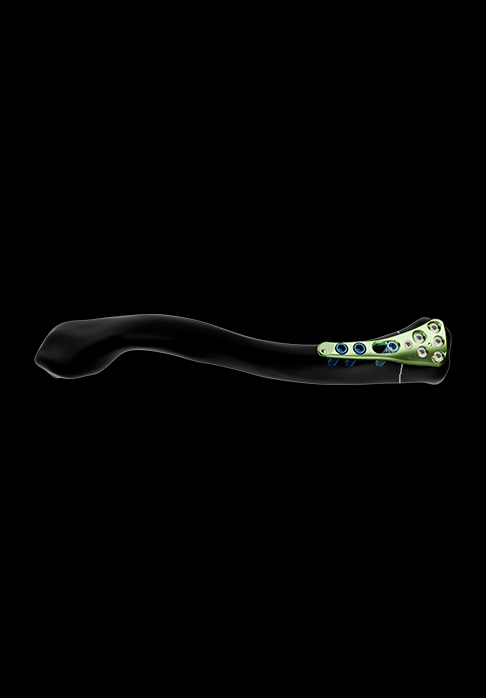
One size of non-malleable, asymmetrical plate is offered for the treatment of medial third fractures.
It features:
- Polyaxial holes for Ø2.8 mm locking screws (in the medial area)
- Oblong hole for Ø3.5 mm non-locking screw
- Holes for Ø3.5 mm locking screws and Ø3.5 mm non-locking screws (in the diaphyseal area)
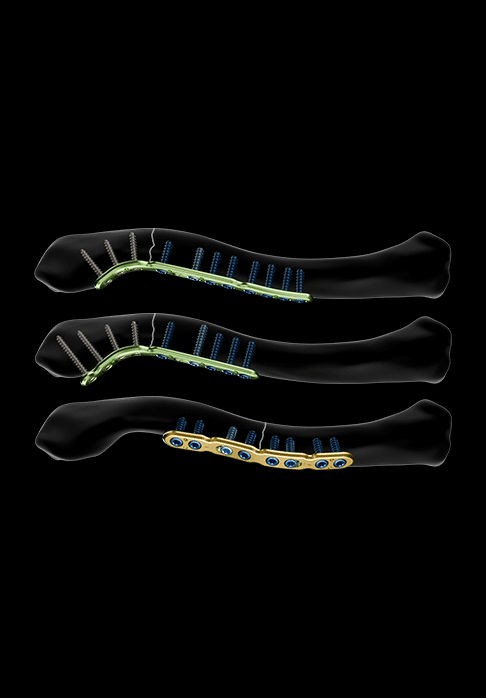
Plates for anterior approach are also available:
- Two variations of anterior plates, malleable and asymmetrical, for laterally displaced middle third fractures (available in size 1):
- The first version features polyaxial holes for Ø2.8 mm locking screws (epiphyseal area) and holes for Ø3.5 mm locking or non-locking screws (diaphyseal area)
- The second version features only polyaxial holes for Ø2.8 mm locking screws
- Two variations of anterior plates, malleable and asymmetrical, for purely lateral fractures (available in size 1):
- The first version features polyaxial holes for Ø2.8 mm locking screws (epiphyseal area) and holes for Ø3.5 mm locking or non-locking screws (diaphyseal area)
- The second version features only polyaxial holes for Ø2.8 mm locking screws
One plate model dedicated to middle third fractures, malleable and symmetrical, with Ø3.5 mm locking or non-locking screw holes (available in size 1).






Key features of the Alians Clavicle S range.
- A range of implants for both superior and anterior surgical approaches
- Multiple plate models offering options adapted to anatomical variations and fracture types, from the medial third to the lateral third
- Dedicated instrumentation designed to meet the specific requirements of clavicle treatment
- Polyaxial locking technology allowing angulation of +/- 10°
- Specific options such as stop drills and a dual guide for butterfly fragment surgery
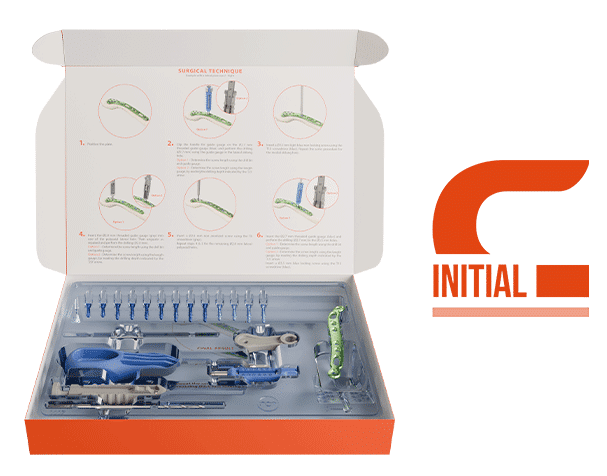
- Available as a sterile, single-use kit with the INITIAL C – Clavicle solution
Our INITIAL solution for the clavicle.
The ONE Platform.
ONE is the patient-specific correction guide solution designed to assist you in the 2D and 3D planning of your surgeries, as well as in performing your osteotomies. The manufacturing of our patient-specific cutting guides is the result of close collaboration between surgeons and Newclip Technics, to best meet surgical needs and support the surgeon throughout every step. These solutions are developed based on clinical imaging data to optimize anatomical congruence between the implant and bone tissue.
Learn more