

The beginning of the 21st century has seen orthopedic lovers focus on developing new approaches to osteosynthesis.
Among them, Grégoire Larché, Jean-Pierre Podgorski and their teams have worked tirelessly within Newclip Technics to develop innovative fixation systems, specific implants, and new solutions, benefiting from modern technologies.
Their ambition remains strong, as they work to contribute to the effectiveness of the surgical procedure, for the best possible patient benefit.
For more than twenty years, joint preservation and bone fracture repair have been the two leitmotifs of the company, allowing patients to recover their mobility.
2002: An idea, a vision,
a reality.
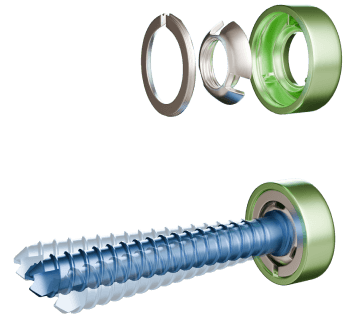
Coming from the world of osteosynthesis, the founders of Newclip Technics initially wanted to improve the fixation system for locking plates. They worked on a technology that combines polyaxiality and locking of the screw to the plate. In 2001, the first polyaxial locking screw-plate fixation system called DualTecSystem (DTS) was created and patented.
This innovation was rewarded at the French Ministry of Research competition, which enabled its launch and large-scale development. The idea then became a reality, and Newclip Technics assumed its status as an independent French osteosynthesis manufacturer, based in the region of Nantes.
Atlantic was released in 2002, the first range comprising implants for knee osteotomy and equipped with the DTS system.
2006 : A new horizon,
the upper limb.
Following the successes achieved with the lower limb, requests were made to transpose this know-how to the upper limb. Since miniaturizing the DTS technology was not enough to meet this challenge, it was necessary to develop a more compact polyaxial locking system (known as DTS2) compatible with implant designs adapted to the anatomical specificities of the bones.
The idea of “bone mapping” was then born. This technique makes it possible to design implants whose bearing surface on the bone takes on the shape of the bone. It also allows an unprecedented congruence of the implant with the bone anatomy, while absorbing the mechanical stresses of the patient’s rehabilitation.
The combination of these different technologies has enabled the creation of other ranges of upper limb-related osteosynthesis implantsfor other joints (elbow, clavicle, etc.).
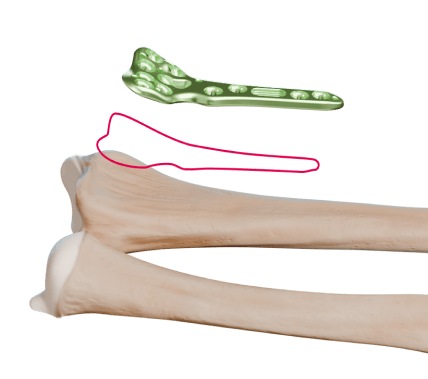
Launched in 2006, Alians Radius was the first upper limb range introduced by Newclip Technics. Designed for wrist fractures, it is continuously evolving over time and with the technologies developed by the company, and is now one of our flagship ranges, known as Xpert Wrist 2.4.
2013 : The single-use sterile kit, a new solution.
Having established its reputation through its fixation systems and implant designs, Newclip Technics was faced with the question of how to optimize the management of high-volume, fast-track osteosynthesis cases in the operating theatre.
Traumatology is synonymous with urgency, and it is sometimes difficult to respond quickly to large patient flows with implants and instruments traditionally offered in containers requiring sterilization in the operating theatre. The handling time and logistics inherent in this type of material naturally lead to a high frequency of patient rotation in the OR.
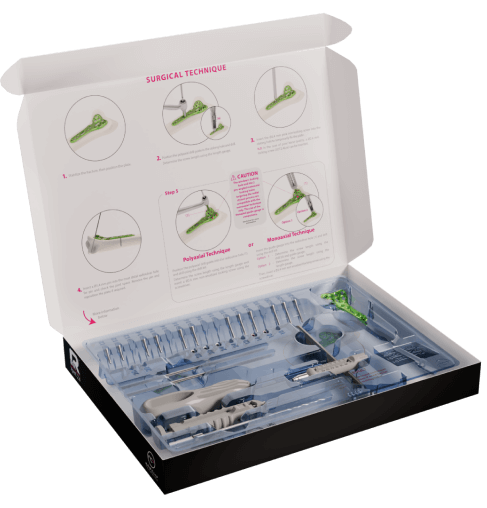
Inspired by sectors such as cardiology and ophthalmology, the INITIAL Solutions concept was launched. It consists of offering single-use sterile kits in blister packs, ready to use, with the latest generation of implants and associated instruments. In 2013, Newclip Technics became the first osteosynthesis company to introduce these kits to the market, with its range for the treatment of wrist fractures: INITIAL R.
Today, this range includes solutions for the clavicle, wrist, hand, knee, ankle, and foot. It has become one of the company’s best-sellers.
2017 : Cutting-edge technology for precision surgery.
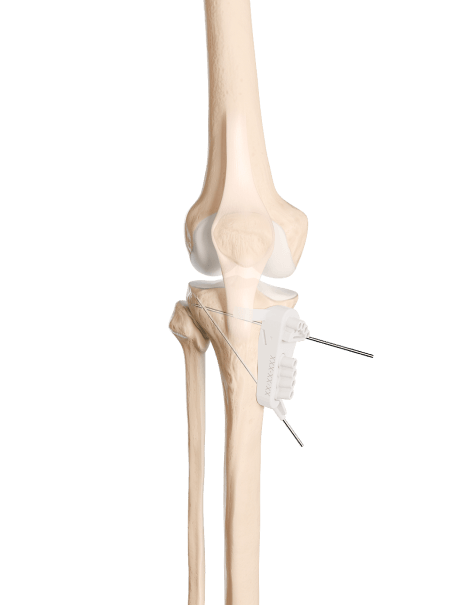
After numerous discussions with surgeons, the question of the accessibility of knee osteotomies arose, as they are operations requiring a certain amount of experience, both in terms of surgical technique and the prior planning required.
Newclip Technics has therefore endeavored to structure and build a genuine service to support the surgeon in these areas. By developing pre-operative simulation solutions applied to the imaging of the patient to be treated, and then by proposing customized instrumentation (cutting guides) intended to faithfully reproduce the previously simulated operation, Newclip Technics has been able to establish a division known as “PSI” (Patient Specific Instrumentation).
With the contribution of 3D printing, its teams of engineers and CAD (Computer-Aided Design) technicians, and its medical imaging operators, Newclip Technics is helping to make complex bone correction procedures accessible and safe. The core of this service is to provide the surgeon with better tools to restore the patient’s functional anatomy as best as possible.
After several years of practice, this PSI division, now called One, is able to offer its know-how in several osteoarticular areas (upper limb, lower limb, foot and ankle, etc.).