The implants of the Activ Fuse range are intended for ankle reconstruction in adults, including the fixation of fractures and arthrodeses of the ankle, distal tibia, talus, and calcaneus.
Activ Fuse enables the performance of a tibiotalar (TT) or tibiotalocalcaneal (TTC) ankle arthrodesis using various approaches in both primary and revision surgeries.
Activ Fuse offers a complete range of plates and screws, designed to accommodate multiple surgical approaches: anterior, posterior, and anterolateral.
The dedicated instrumentation allows for the placement of plates and screws, whether using a standard or minimally invasive approach.
Case Study: Anterolateral construct for TTC fusion.
Solutions for ankle arthrodesis.

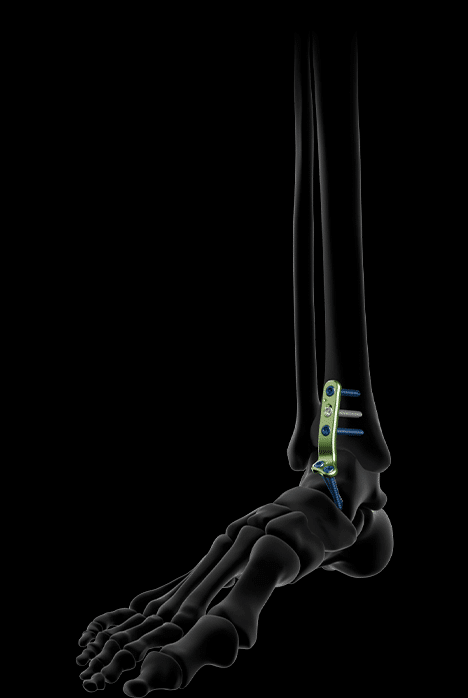

The range of ankle arthrodesis plates for the antero-lateral approach has been designed to preserve the fibula, whenever possible, thanks to its lateralized approach. To accommodate different anatomies, two sizes have been developed.
Each antero-lateral plate features:
- 5 distal pegs to maximize fixation options in the talus
- Two trans-articular screws allowing for TT or TTC arthrodesis. These two trans-articular screws enhance compression at the fusion site.
An optional extension plate extending to the calcaneus, providing additional lateral support during an antero-lateral approach. Its use reinforces TTC arthrodesis stability.

The ankle arthrodesis plate range for the posterior approach has been designed to accommodate the anatomical variations of the posterior malleolus.
Two types of plates have been developed:
- A standard plate and a straight plate, both enabling TTC arthrodesis through the orientation of the trans-articular screw slot
- A plate with three locked holes in the talus, allowing for TT arthrodesis
Each posterior plate includes a trans-articular screw that can be used for TT or TTC arthrodesis. This trans-articular screw passes through the plate to provide compression at the fusion site.




Key features of the Activ Fuse range.
- A comprehensive range of locked plates for performing TT or TTC arthrodesis.
- Designed for 3 different surgical approaches: anterior, anterolateral, and posterior.
- Pre-contoured implants, developed through an original design technique based on bone surface modeling, for optimized anatomical congruence.
- An optional lateral extension to reinforce the initial construct when needed, thanks to its anchorage in the calcaneus.
- A single screw diameter: Ø4.0 mm, available in both locked and non-locked versions.
- Two types of trans-articular screws for TT and TTC arthrodesis: partially threaded compression screws for a lag effect and fully threaded positioning screws to ensure construct rigidity.
- Available in sterile (individual packaging) and non-sterile (within the instrumentation kit) versions.
