Percutaneous foot surgery can be challenging for healthcare professionals. To support surgeons during the procedure, Newclip Technics has developed a targeting guide specifically designed for percutaneous hallux valgus surgery. This guide is used in conjunction with Ø3.5 mm chamfered screws, which are available in sterile or non-sterile versions.
The G•Halva targeting guide is designed to facilitate the positioning and insertion of screws in hallux valgus surgery. Its unique symmetrical design allows similar use for both the left and right sides.
Its modular kit can be ordered directly with Footmotion S screws in a shared container, or combined with the INITIAL S EVO instrumentation kit dedicated to the screws.
Our philosophy: make percutaneous surgery accessible.
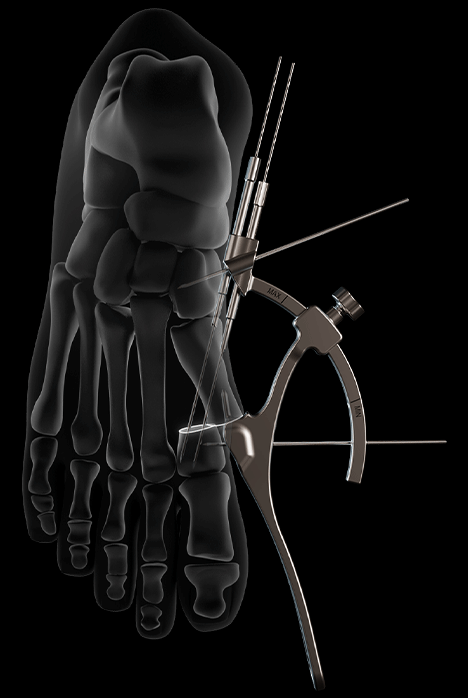
Thanks to its technical features and specific design, the G•Halva guide aims to address the challenges associated with percutaneous surgery.
- Unique symmetrical design, compatible with both left and right foot, allowing one-handed use
- Intra-medullary hook for stable and easy positioning
- M1 head translation combined with M1 shaft translation to lock the TMT1 joint
- Adjustable arch to systematically target the 1st metatarsal head
- Black markings indicating “MIN” and “MAX” positions to prevent interference with the intra-medullary hook
- Guide wire trajectory directed through parallel guides anchored in the proximal first metatarsal

Key features of the G•Halva range
- Unique symmetrical design
- Adjustable arch
- Controlled pin trajectory thanks to parallel guides
- Ergonomic handle
