The implants in the Footmotion Plating System range are intended for arthrodesis, fracture and osteotomy fixation, and foot revision surgeries in adults.
The implants in the Preservation range (included in the Footmotion Plating System) have been specifically designed for medial column and medial arch arthrodesis in cases of collapse.
Two different plate configurations:
– A first configuration for fusion of all four bones in the medial column
– A second configuration dedicated to the medial arch, excluding the talo-navicular arthrodesis, for less severe collapses.
The Preservation range enables treatment of medial arch and medial column collapses, particularly in cases of severe flatfoot or Charcot foot.
Our philosophy.
MEDIAL COLUMN ARTHRODESIS PLATES
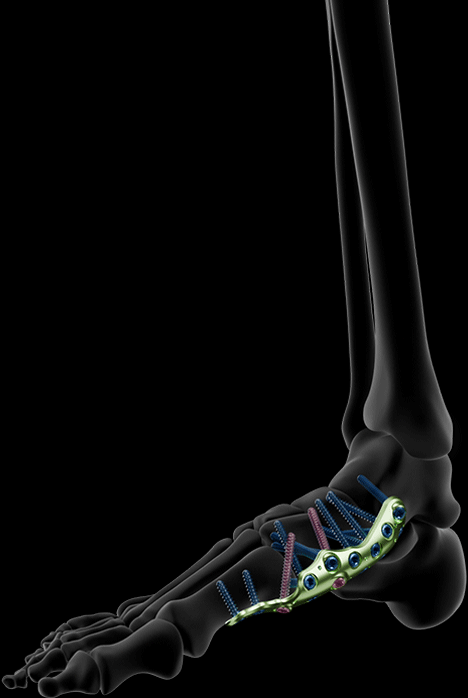
The medial column arthrodesis plates are available in 2 sizes and feature a specifically tiled medio-plantar profile, designed to support the foot arch and provide optimized mechanical properties (compared to a non-tiled profile plate).
The plates include dedicated holes for the insertion of transarticular screws, allowing for compression of each joint.
DISTAL ARCH ARTHRODESIS PLATES
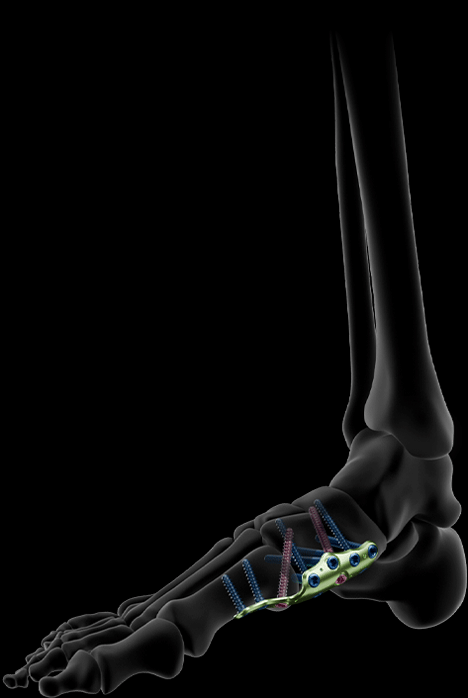
The distal arch arthrodesis plates are available in 2 sizes and feature a specifically tiled medio-plantar profile designed to support the foot arch and provide optimized mechanical properties (compared to a non-tiled profile plate).
This plate excludes the talo-navicular arthrodesis. The plates include dedicated holes for the insertion of transarticular screws, allowing for compression of each joint.


Key features of the Preservation range.
- Screw orientation allows for lateral targeting of the midfoot and posterior anchoring into the talus
- Compatible with both locked and non-locked screws of a single diameter: Ø3.5 mm
- Transarticular screws enable compression across each joint
- Compression and distraction instruments available
