The implants in the Activ Ankle range are designed for the fixation of fractures, osteotomies and pseudarthrosis of the distal and diaphyseal fibula, the distal tibia and the fixation of the syndesmosis in adults.
Activ Ankle is the solution for treating trauma to the internal and external malleolus as well as for diaphyseal fractures of the fibula. Thanks to their design and ease of bending, the plates can respond to a wide spectrum of fractures while allowing, in certain cases, early mobilization of the operated ankle.
Complete treatment of malleolar fractures.
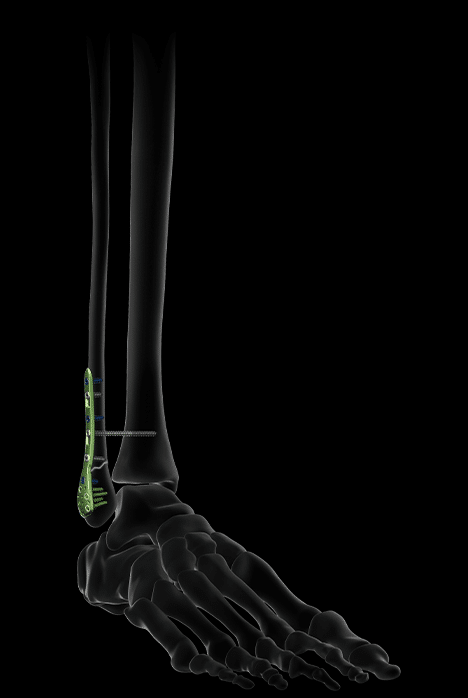
Activ Ankle offers 5 sizes of standard plates for the distal fibula, usable via a lateral approach.
These implants can, if needed, accommodate a syndesmosis screw.
Their distal screws are lockable, polyaxial and Ø2.8 mm, while their proximal screws are Ø3.5 mm, locking or non-locking.
Bending areas also facilitate the congruence of the plate to the bone, in particular on the diaphyseal part, which presents great anatomical variability from one patient to another.
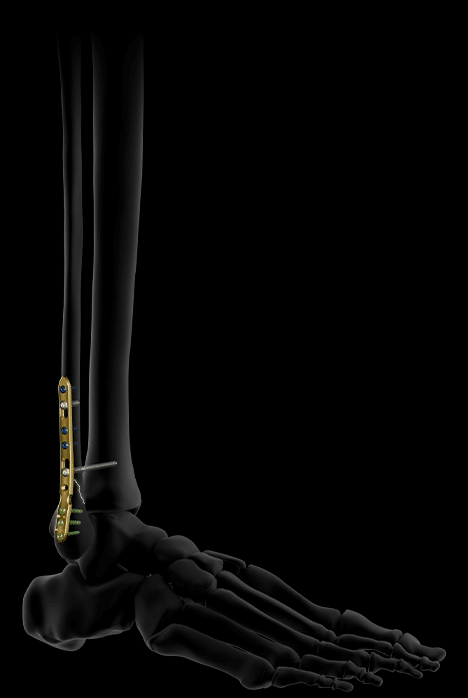
Activ Ankle also offers 2 sizes of narrow lateral plates for distal fibula fractures, designed for use via a lateral approach and capable of accommodating a syndesmosis screw if needed.
Their distal screws are lockable, polyaxial and Ø2.8 mm, while their proximal screws are Ø3.5 mm, locking or non-locking.
Bending areas facilitate the congruence of the plate to the bone, in particular on the diaphyseal part, which presents great anatomical variability from one patient to another.
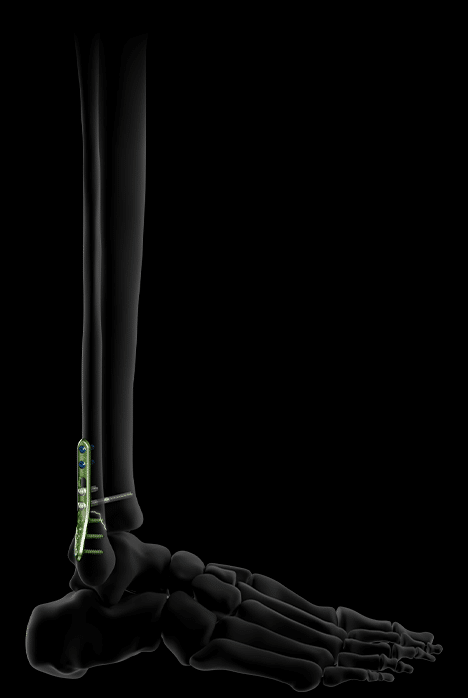
Activ Ankle also offers 3 sizes of plates for treating distal fibula fractures via a posterolateral approach.
Their distal screws are lockable, polyaxial and Ø2.8 mm, while their proximal screws are Ø3.5 mm, locking or non-locking.
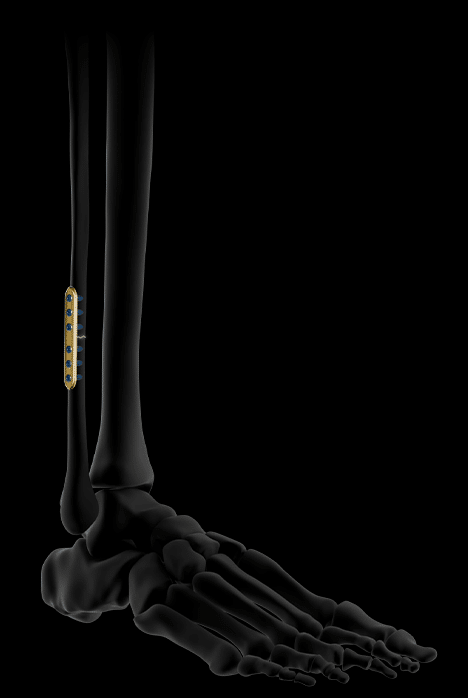
It accepts Ø3.5 mm locking and non-locking screws.
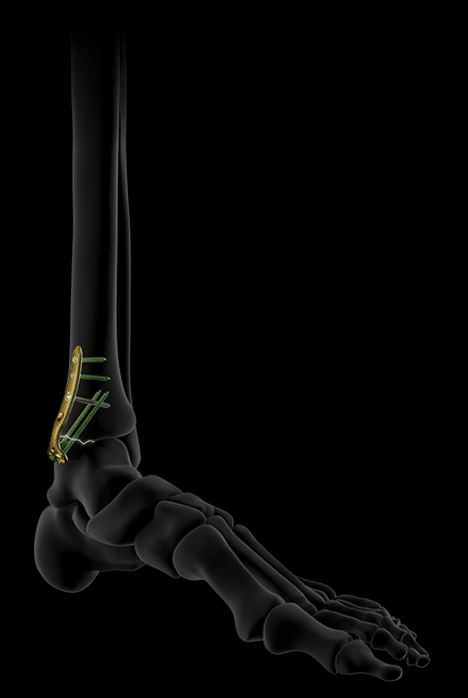
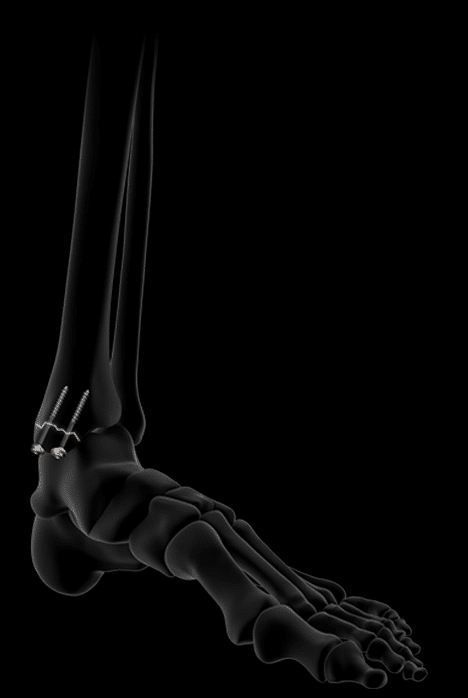






Key features of the Activ Ankle range.
- A range of plates for three surgical approaches
- Angular freedom of 20° to ensure locked polyaxial fixation of distal screws
- ØØ3.5 mm cortical screw or Ø4.0 mm lag screw, available for fixation of the syndesmosis
- Implants available in sterile or non-sterile versions or in a single-use kit (Initial A)
Our INITIAL solution for the ankle.

